Flexible and Efficient:
Committed to Advancing Vaccine Process Optimization
Compared to traditional inactivated or live attenuated vaccines, new vaccines demonstrate outstanding immune efficacy, minimal side effects, higher yields, and batch-to-batch stability, making them more favored by current vaccine manufacturers. These novel vaccines include viral vector vaccines based on cell culture and virus amplification, recombinant protein vaccines expressed in prokaryotic or eukaryotic cells, and mRNA vaccines based on in vitro transcription, among others.
Assisting current vaccine manufacturers in producing cost-effective yet rigorously compliant vaccines that meet production quality standards has become a comprehensive test of upstream technology and service providers’ capabilities.
LePure Biotech, with a focus on research and development, offers flexible process solutions for viral vector vaccines, mRNA vaccines, and inactivated vaccines to accelerate process optimization and is dedicated to providing comprehensive vaccine process solutions for vaccine manufacturers.
General Process Steps of Viral Vaccine
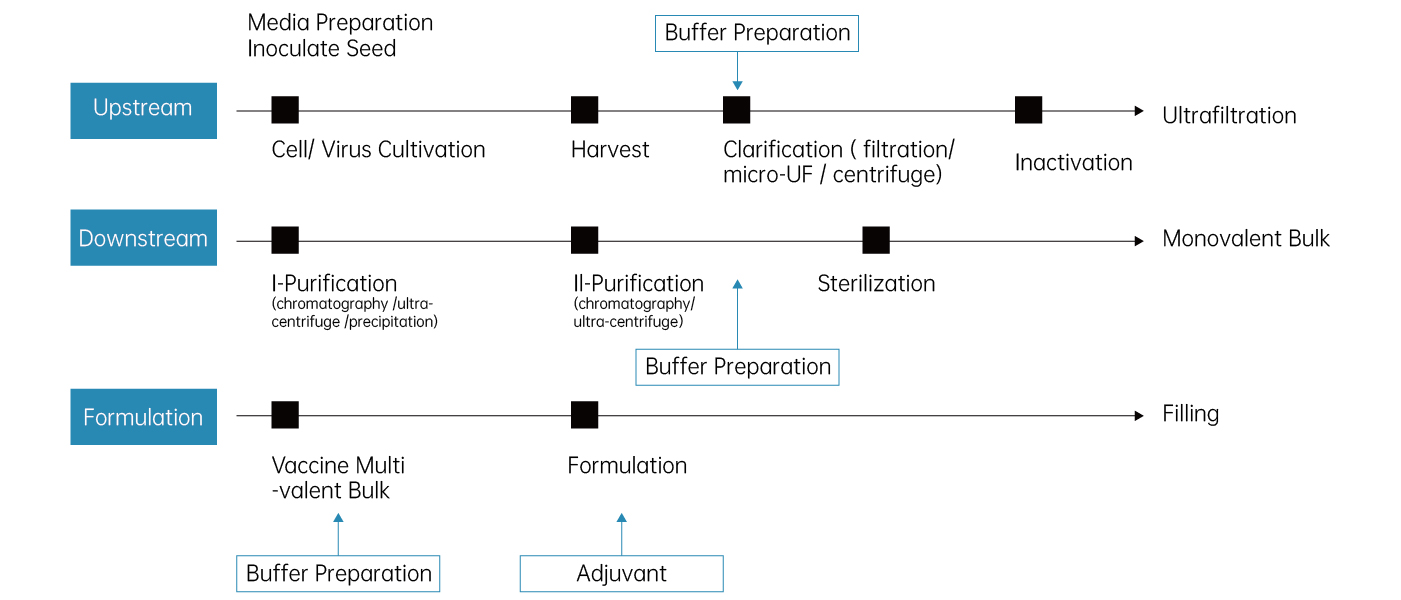
Cell Culture
LePure Biotech’s cell culture media provides an ideal environment for cell growth and proliferation, while our bioreactors and fermentation equipment, essential tools in cell cultivation, contribute to the enhancement of virus propagation and protein expression.
Filtration & Purification
Our professional filters and filtration application not only help maintain the cleanliness of the culture media but also ensure the high purity of the vaccine liquid. Simultaneously, our separation and purification equipment play a critical role in accurately extracting target components, ensuring the quality of the final vaccine.
Transport & Formulation
Cold chain equipment plays a vital role in the storage and transportation of vaccines, maintaining stability at various stages. Additionally, our biostatistics tools in the vaccine design and development process provide advanced data analysis and monitoring, promoting precise vaccine design and efficient production.
Pursuing Professional Perfection
Our expertise ensures an efficient and high-quality production process, contributing to the rapid development of both traditional and novel vaccines. Based on product quality risk management, we establish a quality system that complies with NMPA, ICH, FDA, EU regulations, and guidelines to meet the requirements of the entire drug life cycle management, ensuring regulatory compliance for product registration applications.