Transforming Molecules into Cutting-Edge Films:
LePure Biotech’s Innovations in Biopharmaceutical Consumables
In recent years, the biopharmaceutical industry has witnessed a remarkable surge in the demand for single-use consumables. However, this surge has been met with significant challenges due to inadequate production capacity and an unstable supply chain, resulting in a critical global shortage of disposable consumables.
In response to this complex technical conundrum, we are proud to introduce the LeKrius Bioprocess film, a comprehensive solution that tackles the issue at its core, spanning from raw material formulation to coextrusion processes. Every aspect of the LeKrius Bioprocess film is designed and manufactured by LePure Biotech, effectively alleviating the shortage of single-use consumables.
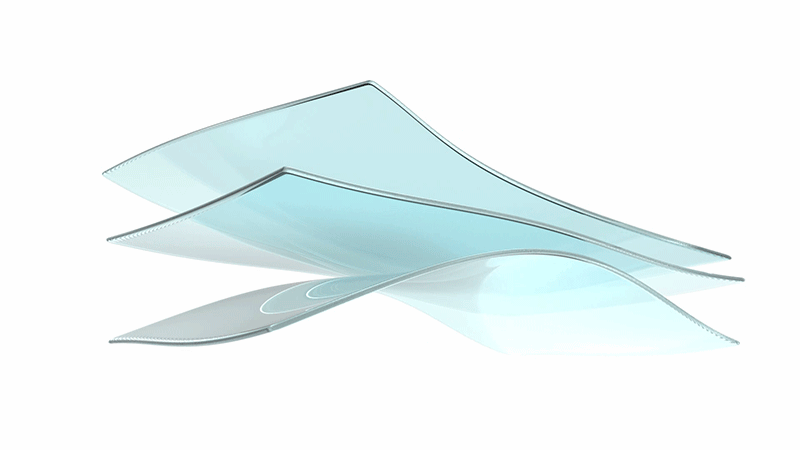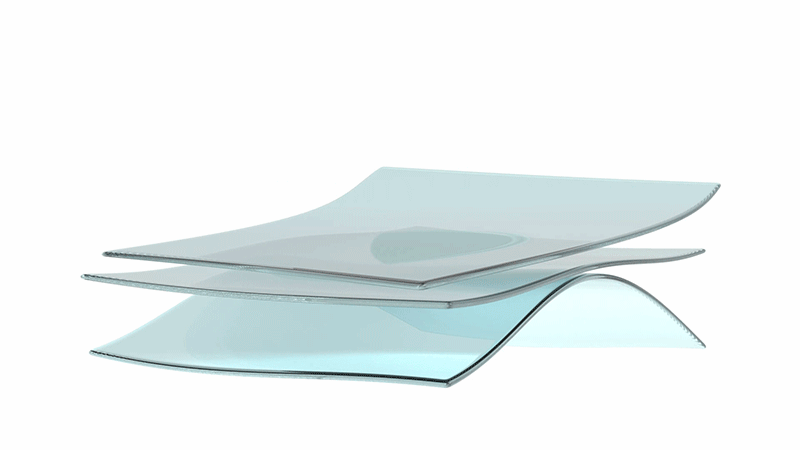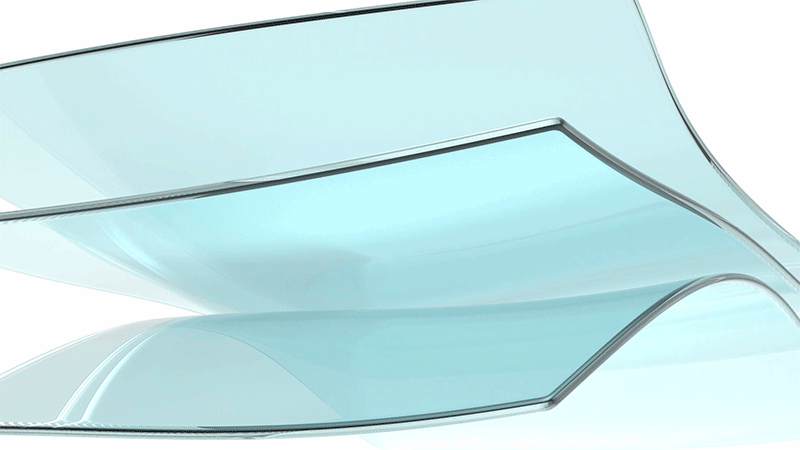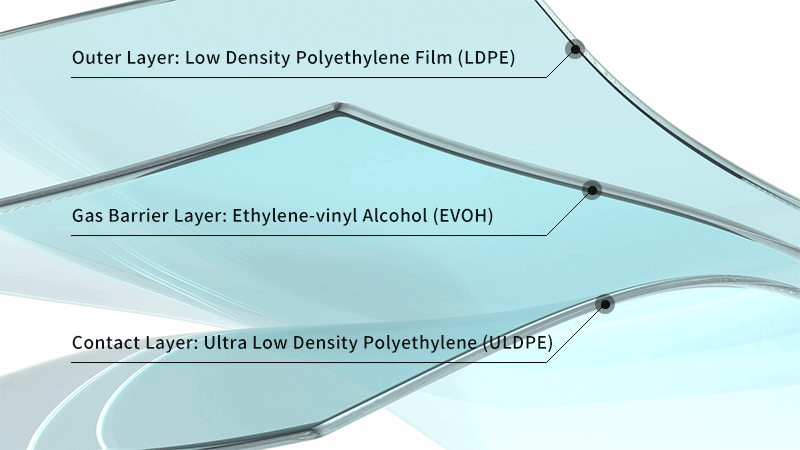
- More Flexible: Developed independently by LePure Biotech, the LeKrius membrane has a thickness of 400μm, surpassing other membranes on the market.
- More Durable: The welding process of the LeKrius single-use bioprocess film has undergone real-time monitoring to ensure process stability.
- Better Compatibility : Strict testing during incoming material inspection ensures dimensions within tolerance ranges.
- 100% Integrity Testing : Each LeKrius single-use bag undergoes 100% integrity testing for higher detection sensitivity.
More Features
- Enhanced Customization:
Tailoring Single-Use Bags to Your Unique Requirements - Dependable Supply Chain:
Global Production Capacity Ensures On-Time Delivery
-
Unparalleled Purity:
LePure Biotech’s Pioneering “Ultra-Clean Process Patent” -
Integrity Guarantee:
One-Way Material Flow to Prevent Cross-Contamination
-
Established Quality:
Over 12 Years of Proven Excellence in Single-Use Systems -
Efficient Validation Services:
Swift and Secure In-House Single-Use System Validation
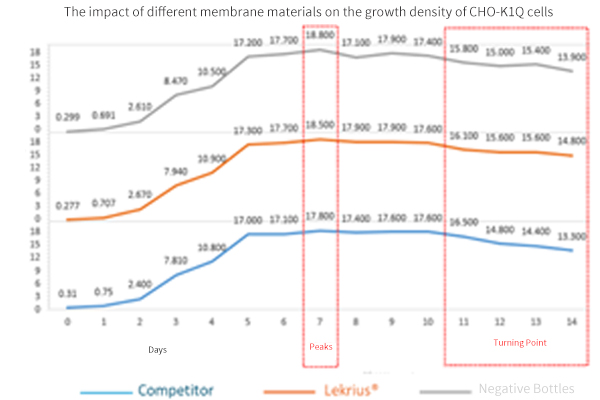
- Experiment Objective:
Test the impact of different membrane materials on the growth density and cell viability of CHO K1.
- Experimental Parameters:
Cell Types: CHO K1
Culture Medium Extraction Conditions: 37°C, 3 days
Area-to-Volume Ratio: 3 cm²/mL
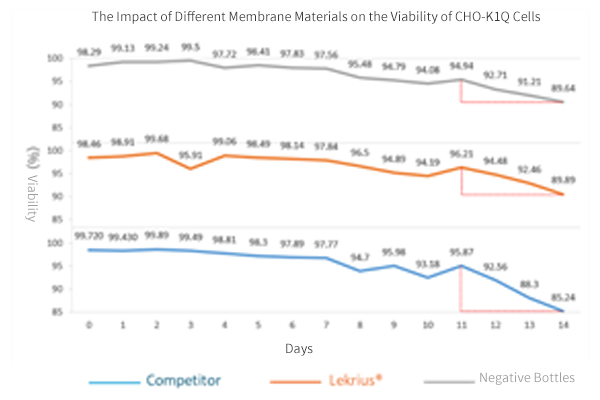
- Experimental Membrane Models:
LeKrius Membrane VS Competitor, negative bottles control
- Conclusion of CHO K1 Cultivation Experiment:
Live cell density is generally comparable to bottle cultivation, superior to the Competitor.
Over the 14-day cell culture period, cell viability remains close.
The use of single-use systems involves a variety of raw materials, such as biopharmaceutical films, various connectors, and multiple types of tubing, among others.
LePure Biotech conducts incoming material inspections for all raw materials, ensuring product quality and supply chain security from the source. We have systematically categorized raw materials and established corresponding inspection criteria.
| Test Standard | Test Result |
|---|---|
| USP <85> | √ |
| USP <87> | √ |
| USP <88> | √ |
| USP <661> | √ |